| Chemical Formula | HeH+ |
| Molar Mass | 5.01054 g/mol |
Synthesis
In the laboratory, helium hydride is produced by bombarding a low pressure hydrogen-helium mixture with electrons. Although helium hydride has been detected in space, not much is known about its natural formation.
Structure
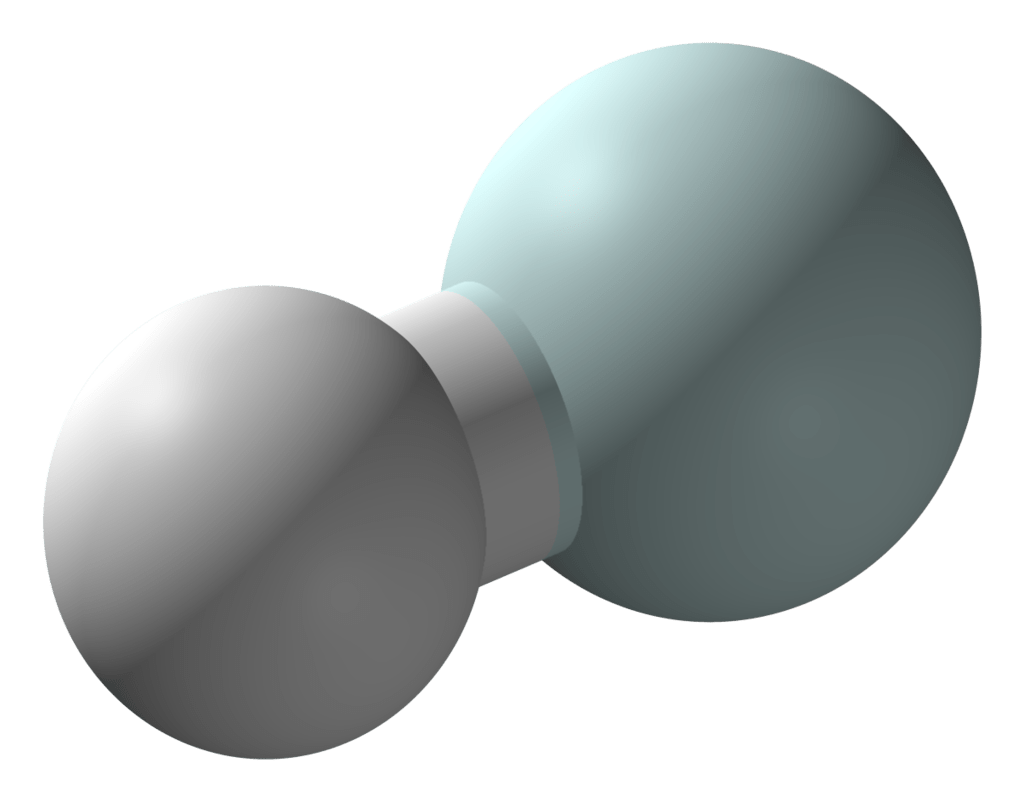
Source: https://en.wikipedia.org/wiki/Helium_hydride_ion#/media/File:Helium-hydride-cation-3D-balls.png
The structure is quite simple, since it is a linear molecule of two atoms. It consists of a neutral helium atom at one end and a positively charged hydrogen atom in the other. Having only two electrons, it’s as if the helium atom is donating its electron to the hydrogen atom in order to neutralize it.
History and Trivia
Scientists first knew about helium hydride when it was synthesized in a laboratory in 1925 by T.R. Hogness and E.G. Lunn using the method mentioned above. Because it contains a positively charged hydrogen atom, they hypothesized that it was a strong acid: it would donate the hydrogen atom to most substances it comes in contact with. However, its physical and chemical properties are not yet well studied, so this is still yet to be proven.
But the existence of helium hydride dates back all the way to 380,000 years after the Big Bang. In fact, some scientists even think that it’s the first compound that ever existed. Since helium and hydrogen were one of the first elements to form, the early conditions of the Universe allowed helium to collide with a proton (which is the same thing as a positively charged hydrogen atom), hence forming helium hydride. This reaction releases energy in the form of photons.
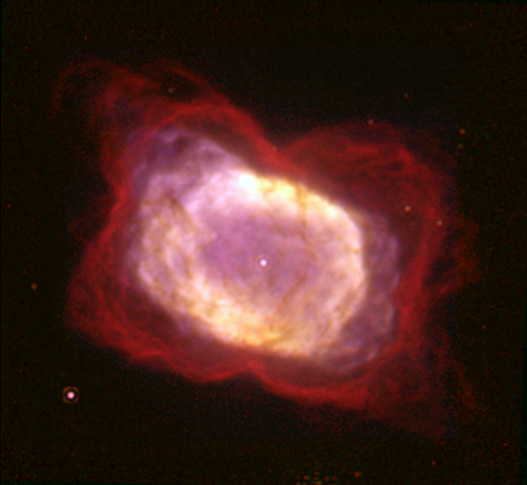
Helium hydride is thought to hide in nebulae and early regions of our galaxy, floating around while remaining intact. This was conjectured in the 1970s, but was only confirmed recently in 2019 by Rolf Güsten and his colleagues from the Max Planck Institute for Radio Astronomy.
Source: https://en.wikipedia.org/wiki/NGC_7027#/media/File:NGC_7027HSTFull.jpg
Güsten and his colleagues pointed an airplane-based telescope at the planetary nebula NGC 7027. Using tetrahertz spectroscopy, they were able to detect the spectrum of helium hydride, finally confirming the hypothesis that remained unanswered for nearly 50 years. As of now, more research needs to be done on helium hydride in order to determine its other properties, such as acidity and reactivity.
References
ACS [Internet]. c2019. Washington, DC: American Chemical Society [cited 18 June 2020]. Available from: https://www.acs.org/content/acs/en/molecule-of-the-week/archive/h/helium-hydride.html.
Güsten R, Wiesemeyer H, Neufeld D, Menten KM, Graf UU, Jacobs K, Klein B, Ricken O, Risacher C, Stutzki J. 2019. Astrophysical detection of the helium hydride ion HeH+. Nature 568(7752): 357-359.
Hogness TR, Lunn EG. 1925. The Ionization of Hydrogen by Electron Impact as Interpreted by Positive Ray Analysis. Phys. Rev. 26(1): 44-55.