| Molecular Formula | C55H74MgN4O6 |
| Molecular Weight | 911.5 g/mol |
| Color | Light green |
Structure
The “head” portion (encircled in red) of the bacteriochlorophyll consists of a partially hydrogenated porphyrin ring known as chlorin. It contains a central magnesium atom. Meanwhile, the “tail” portion (encircled in blue) is a hydrocarbon chain, connecting to the head by an ester (encircled in yellow).
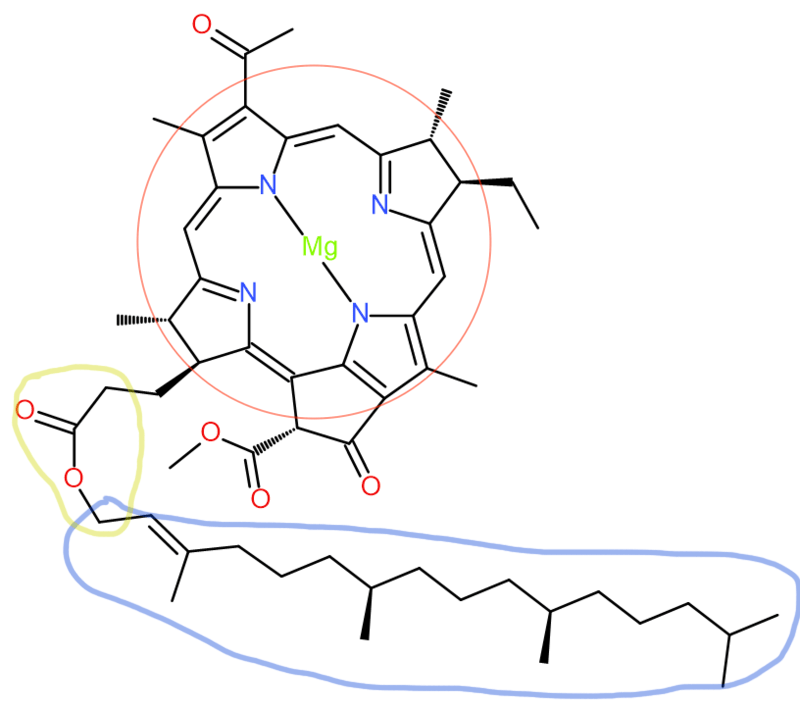
Edited from: https://en.wikipedia.org/wiki/Bacteriochlorophyll#/media/File:BacterioChlorophyll_a.svg
Synthesis
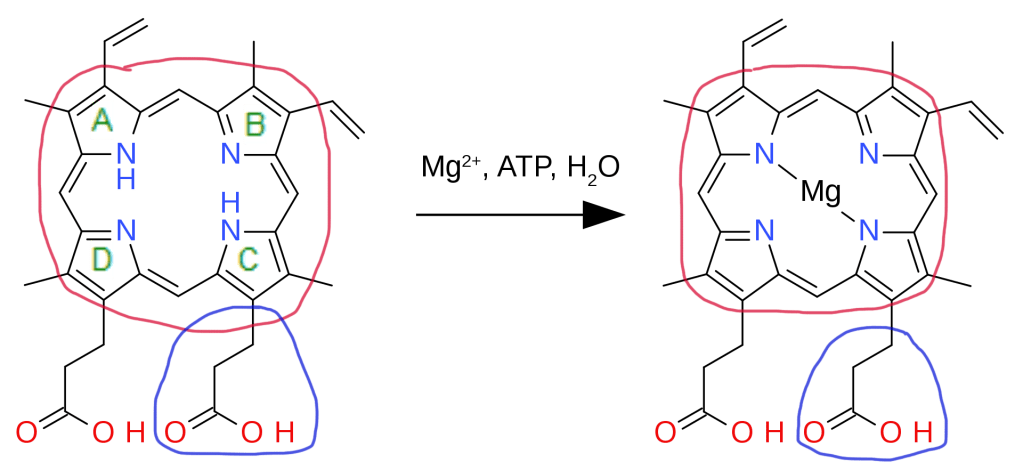
Edited from: https://en.wikipedia.org/wiki/Chlorophyllide#/media/File:Protoporphyrin_magnesium_insertion.svg
Bacteriochlorophylls, as its name suggests, are a type of chlorophyll produced by photosynthetic bacteria. The biosynthesis of bacteriochlorophyll a in particular begins with protoporphyrin IX previously synthesized from glutamyl tRNA. Magnesium is first inserted into protoporphyrin IX (encircled red) with the help of ATP in an aqueous medium. This reaction is catalyzed by the enzyme magnesium chelatase.
Focus on the group of atoms on the lower right attached to the main ring, encircled blue (a carboxylic acid). The hydrogen is removed and a methyl chain is added (CH3). This reaction is aided by the enzyme Magnesium protoporphyrin IX methyltransferase.
Keep your attention on the previously modified group of atoms that are attached to the main ring, encircled blue (now an ester). With the help of three electron donors known as nicotinamide adenine dinucleotide phosphate (NADPH), the enzyme Magnesium-protoporphyrin IX monomethyl ester (oxidative) cyclase adds three H+ ions and an O2 molecule. This greatly alters the structure of the ester branch into a group of atoms with a very different geometry, but it continues to cling on to the porphyrin ring (encircled yellow). The resulting product is protochlorophyllide.
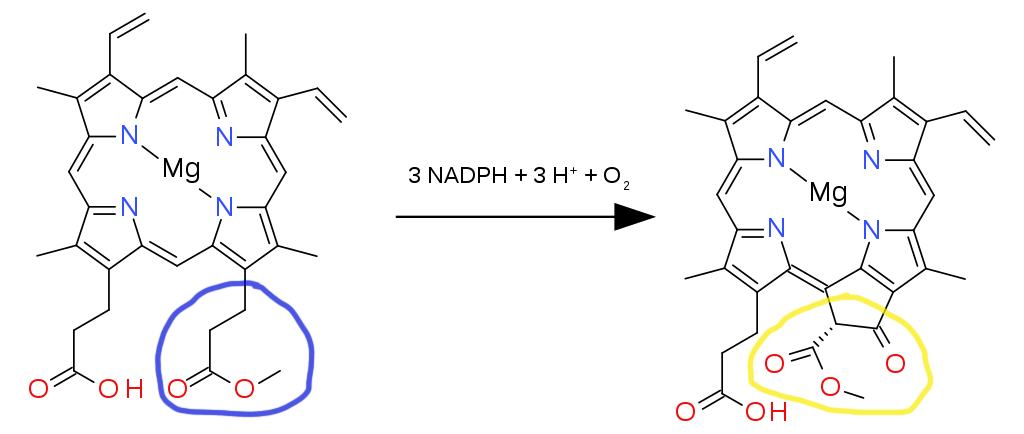
Edited from: https://en.wikipedia.org/wiki/Chlorophyllide#/media/File:Oxidative_cyclase_to_chlorin.svg
The chlorin is then converted into a chlorophyllide a using the enzyme protochlorophyllide reductase. The reaction still requires the same electron donor NADPH. As the electron is donated to an H+ ion to become a hydrogen atom, this same neutral hydrogen atom is added to the molecule to slightly change its structure.

Edited from: https://en.wikipedia.org/wiki/Chlorophyllide#/media/File:Chlorophillide_synthesis.svg
Finally, on the carboxylic acid group on the chlorophyllide a product encircled in green, the hydrocarbon chain is attached by a series of reactions using ATP, water, and H+ ions carried out by a series of enzymes. The end product is bacteriochlorophyll a, as depicted in Fig. 1. For bacteriochlorophyll b-g, the process is similar, only with slight modifications involving how specific enzymes modify the structure of the molecule.
Uses
Bacteria such as Chlorobium tepidum use bacteriochlorophyll in order to harvest light energy in the form of photons for photosynthesis. Similar to the process found in non-bacterial chlorophylls, the incoming energy from the photons liberates electrons from the molecule. This allows the electrons to be bounced and passed from one bacteriochlorophyll to another until it finally finds its destination, the reaction center.
Many bacteria use bacteriochlorophyll in anoxygenic photosynthesis, which produces not oxygen (O2), but sulfur (S). It also requires hydrosulfuric acid (H2S) as a reactant rather than water (H2O).
Trivia
Bacteriochlorophyll are found in Fenna-Matthews-Olson (FMO) complexes, wrapped around by a protein scaffold. These FMO complexes, with the help of the bacteriochlorophylls, are theorized to use quantum light harvesting in order to efficiently use the energy obtained from incoming photons.

Source: https://en.wikipedia.org/wiki/Fenna-Matthews-Olson_complex#/media/File:Fenna-Matthews-Olson_complex_protein_trimer_(PDB_cartoon_4bcl).png
Because the electron needs to jump from bacteriochlorophyll to bacteriochlorophyll before it reaches the reaction center, the electron can eventually get lost along with the energy it carries with it. But experiments show that photosynthesis happens at nearly 100% efficiency.
According to quantum mechanics, the electron is both a particle and a wave. Thus, due to its wave-like properties, it can be in several places at once as it is spread out across the material – a superposition of locations. But being a particle, one can only observe it to be in one place. Hence, the wave is interpreted as a sort of probability distribution of where the particle might be.
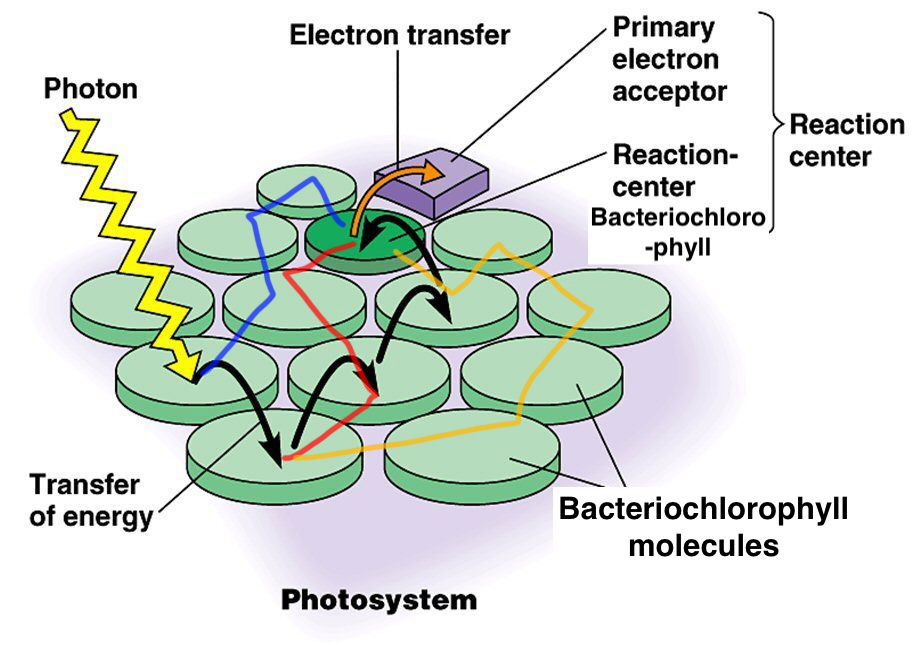
Edited from: https://alevelbiologystudent.weebly.com/173-photosynthesis.html
This can also be interpreted as the electron traveling an infinite number of possible paths at the same time as it covers all locations. Thus, according Engel (2011), since it takes all possible paths at the same time, it can find its destination without losing any energy.
References
Urry LA, Cain ML, Minorsky PV, Wasserman SA, Reece JB. 2017. Campbell Biology 11th Edition. New York City (NY): Pearson Education. 1284 p.
Engel GS. 2011. Quantum coherence in photosynthesis. Procedia Chemistry 3 (2011): 222-231.
PubChem [Internet]. c2020. Bethesda, MD: National Center for Biotechnology Information [cited 2020 June 14]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Bacteriochlorophyll-a