| Molecular Formula | C34H34N4O4 |
| Molecular Weight | 310.4 g/mol |
| Solubility | 169 mg/L |
Structure
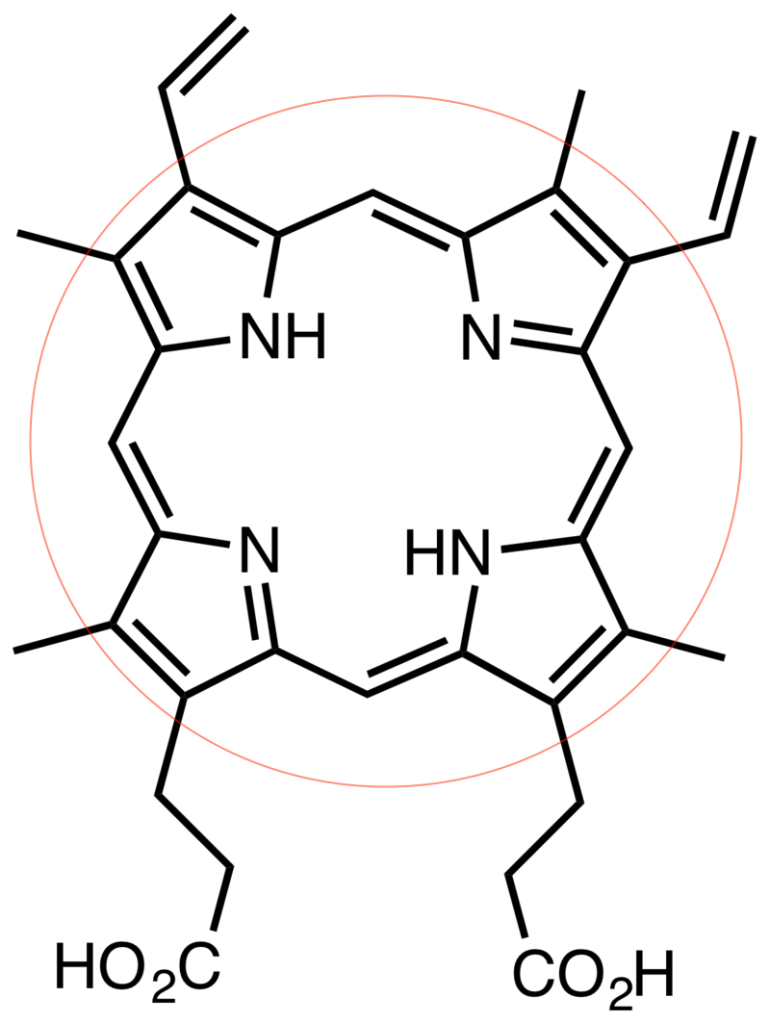
Protoporphyrin IX falls under a special type of cyclic compounds: they are macrocycles, or molecules that have a ring of twelve or more atoms. The main bulk of the molecule is called a porphine core, encircled in red in Fig. 1.
Synthesis
Biosynthesis begins with the formation of 5-aminolevulinic acid, which is synthesized from either glycine and succinyl-CoA from the citric acid cycle (usually in non-photosynthesizers) or from glutamyl tRNA (usually in photosynthesizers). But the process is the same for all organisms from there, shown below in Fig. 2.
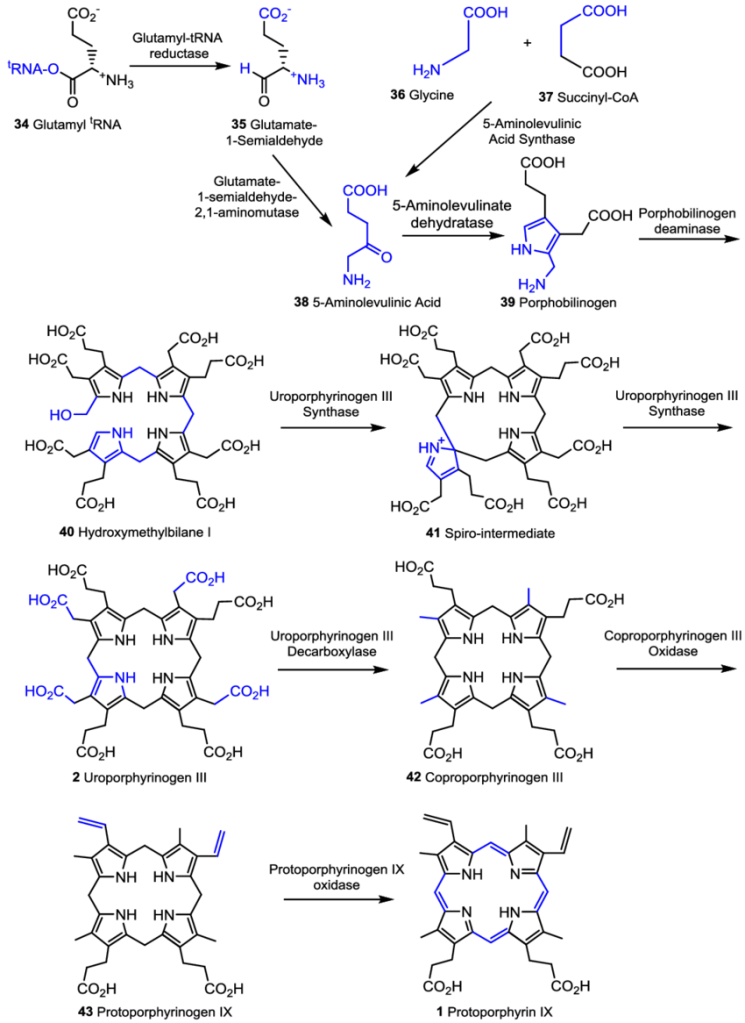
Source: https://www.researchgate.net/figure/Biosynthesis-of-protoporphyrin-IX_fig10_265489344
Uses and Trivia
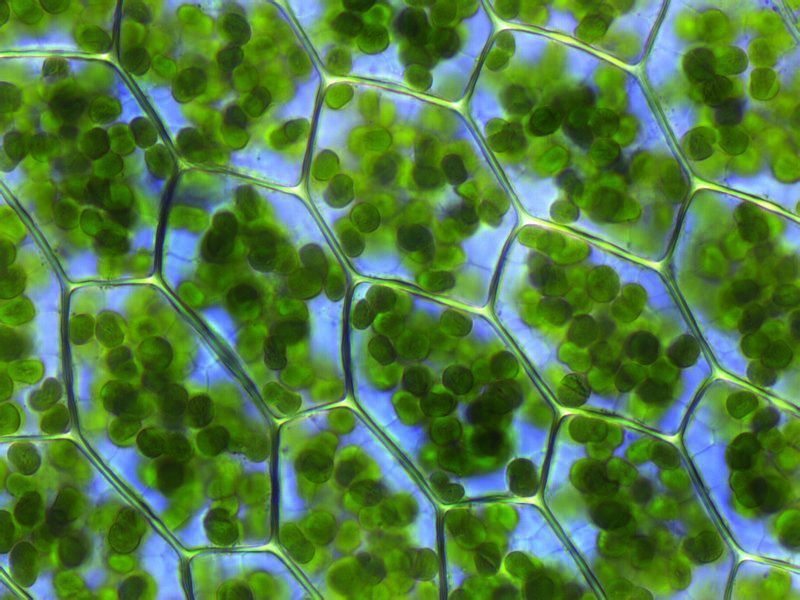
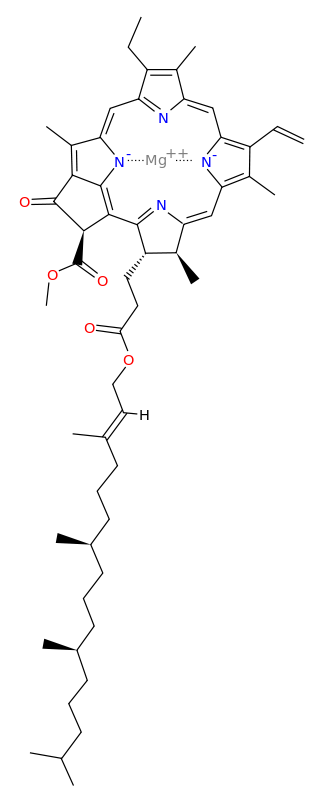
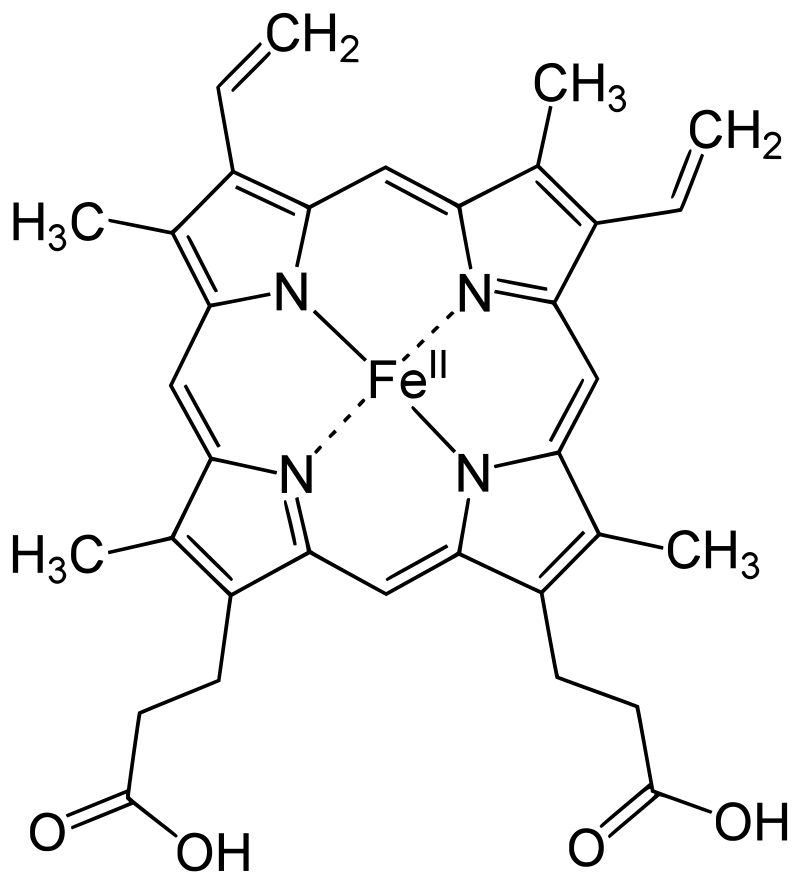
Protoporphyrin IX is a versatile molecule that is used to synthesize a variety of molecules such as heme and chlorophylls. Heme is a red pigment found in the blood and is used for oxygen delivery to body tissues. Chlorophyll (a and b) on the other hand are green pigments found in the chloroplasts of plant cells used for harvesting light energy. It’s interesting to see how both of these molecules of different color, origin, and use originate from the same substance!
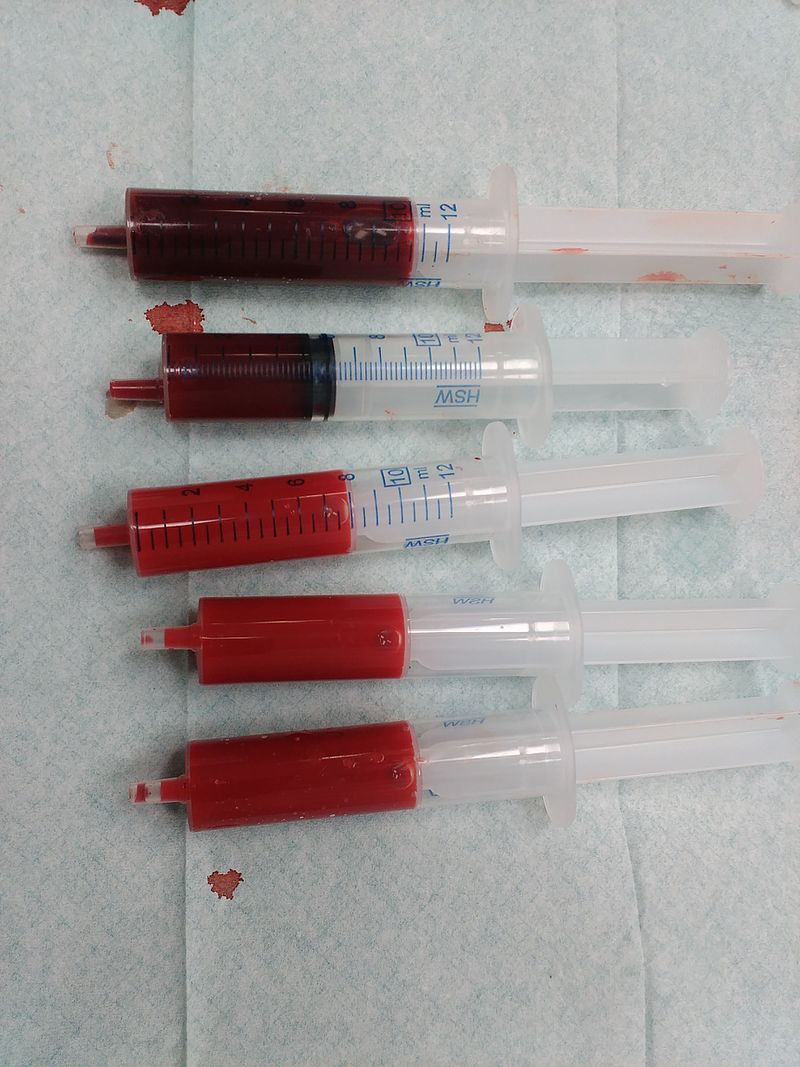
Figure 3. Left to right: (a) Chloroplasts with a green chlorophyll pigment, (b) structure of chlorophyll a, (c) blood with red heme pigment (bright red – heme has oxygen bound to it, dark red – heme has no oxygen bound do it), (d) structure of heme.
Protoporphyrin IX is also used in photodynamic therapy, which is a technique to treat cancer. In this treatment, a photosensitizer – in this case, protoporphyrin IX – is made toxic by activating it with a specific wavelength of light. The toxicity of the photosensitizer can destroy cancerous and precancerous cells.
References
Battersby AR, Fookes CJR, Matcham GWJ, McDonald E. 1980. Biosynthesis of the pigments of life: Nature 285(1): 17-21.
PubChem [Internet]. c2020. Bethesda, MD: National Center for Biotechnology Information [cited 2020 June 12]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Porphyrin
Sachar M, Anderson KE, Ma XC. 2016. Protoporphyrin IX: the Good, the Bad, and the Ugly. J Pharmacol Exp Ther. 356(2): 267-275.
Senge MO, Ryan AA, Letchford KA, Macgowan S. 2014. Chlorophylls, Symmetry, Chirality, and Photosynthesis. Symmetry 6(3): 781-843.
One thought on “Protoporphyrin IX”